A world’s first: crystalline capsules that release their contents upon irradiation with light. Developed by Professor Kingo Uchida’s research group (Faculty of Advanced Science and Technology) and published in Chemical Science, a journal of the Royal Society of Chemistry
2021.08.23
Molecular crystalline capsules that release their contents by light
Professor Kingo Uchida’s research group (Faculty of Advanced Science and Technology), which conducts research into photoresponsive organic crystals, has succeeded in creating the world’s first crystalline capsule that releases its contents upon irradiation with light.
The phenomenon of photosalience whereby organic crystals fragment when irradiated with light has been a topic of recent interest, and this marks the world’s first report of a system that uses the recrystallization method to grow crystals of an organic compound, combines a photoreactive material and solvent with a third substance to create crystals that encapsulate the third substance, and breaks the capsule and releases its contents by irradiating the capsule with light.
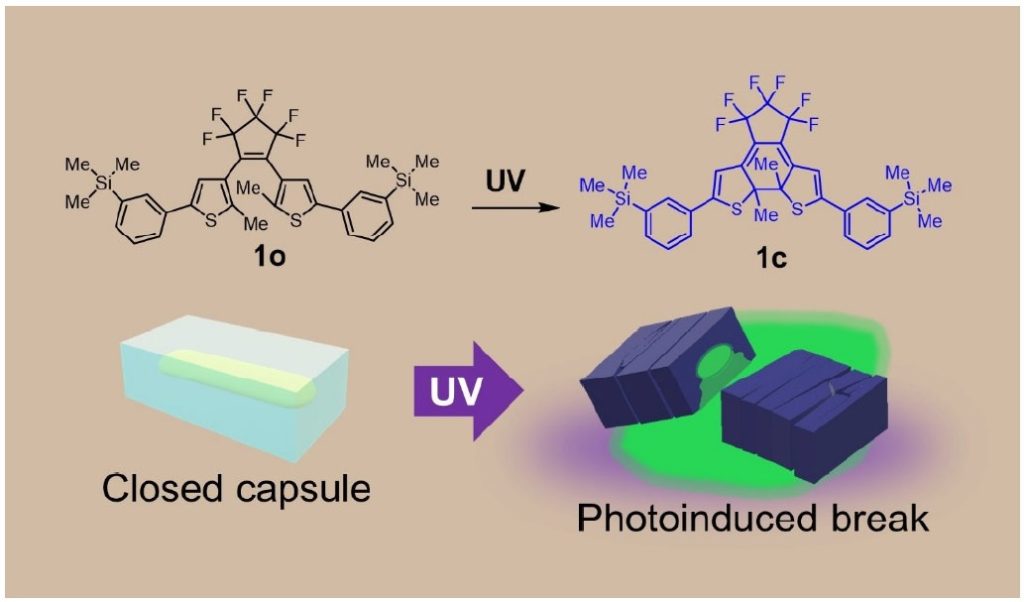
Through a trial and error process utilizing different crystalline capsule-forming molecular structures, molecule 1o (where “o” is taken from “open-ring isomer”) produced crystal capsules with a 7.7 % yield using a normal recrystallization method of growing organic crystals in solvent.
When the research group tried dissolving a substance to be encapsulated and DAE in an organic solvent, it created DAE capsules that encapsulated the substance. For easy identification, the group selected a fluorescent dye called fluorescein that emits green fluorescent light as the substance to be encapsulated. A mixture of this fluorescent dye and DAE 1o were dissolved in alcohol and left to stand at room temperature, and as the solvent evaporated, crystalline capsules of 1o were created that encapsulated an alcohol solution of fluorescein with 21 % yield. When irradiated with ultraviolet light, the crystalline capsules then shattered due to photosalience and green fluorescent light appeared around the capsule.
Notably, the size of these crystalline capsules can be reduced by using shorter recrystallization times.
The ability to release encapsulated chemical substances with light shows promise in various applications, including optical fuses.
The findings of this research have been published in Chemical Science, the flagship journal of the Royal Society of Chemistry.
[Presented Paper]
Title: Molecular crystalline capsules that release their contents by light
Journal: Chemical Science
https://doi.org/10.1039/D1SC03394H
Authors:
- Akira Nagai (Department of Materials Chemistry, Faculty of Science and Technology, Ryukoku University)
- Ryo Nishimura (Department of Materials Chemistry, Faculty of Science and Technology, Ryukoku University)
- Yohei Hattori (Materials Chemistry Course, Faculty of Advanced Science and Technology, Ryukoku University)
- Eri Hatano (Department of Materials Chemistry, Faculty of Science and Technology, Ryukoku University)
- Ayako Fujimoto (Department of Materials Chemistry, Faculty of Science and Technology, Ryukoku University)
- Masakazu Morimoto (Department of Chemistry and Research Center for Smart Molecules, Rikkyo University)
- Nobuhiro Yasuda (Japan Synchrotron Radiation Research Institute)
- Kenji Kamada (Nanomaterials Research Institute (NMRI), National Institute of Advanced Industrial Science and Technology (AIST))
- Hikaru Sotome (Graduate School of Engineering Science, Osaka University)
- Hiroshi Miyasaka (Graduate School of Engineering Science, Osaka University)
- Satoshi Yokojima (School of Pharmacy, Tokyo University of Pharmacy and Life Sciences)
- Shinichiro Nakamura (Nakamura Laboratory, RIKEN Cluster for Science, Technology and Innovation Hub)
- Kingo Uchida (Materials Chemistry Course, Faculty of Advanced Science and Technology, Ryukoku University)
- A stable radical exhibiting high fluorescence efficiency in solution has been developed by the research group of Professor Kingo Uchida and Assistant Professor Yohei Hattori (Faculty of Advanced Science and Technology) and published in Chemical Science, a journal of the Royal Society of Chemistry
- All
- Kingo Uchida’s research work recognized by Research.com 2023 best scientist ranking in the chemistry category